Collaborative Extraction
A Fully Integrated Extraction and Extract Treatment Process
Novel polarity-directed and mixed-polarity CO2 extraction solvent systems and processes utilizing combinations of dense phase CO2, water, natural and eco-safe (and minimized) organic compounds and inorganic additives, solid or liquid extractable substances, CO2 expansion and salting-out assisted liquid-liquid extraction (CO2 SALLE), and optimized extraction process energies.
Referring to Figure 1, a semi-aqueous infusion ❶ comprising fermented ethanol, water, and carbonic acid (functioning as a polar co-extractant) is quantitatively CO2 expanded and extracted from a semi-aqueous solution (SAS), for example an organic grain alcohol, and mixed into purified liquid CO2 (functioning as a nonpolar bulk carrier solvent and co-extractant) in a process called CO2 Expansion/Salting-Out Assisted Liquid-Liquid Extraction (CO2 SALLE). CO2 SALLE produces a solvent mixture having a nonpolar-polar-ionized solubility gradient (↕δ), enabling full spectrum extraction ❷ of finely ground plant material, and providing fractional (carbonic acid catalyzed) decarboxylation in-situ of Liquid CO2:EtOH:H2CO3:Extract mixtures.
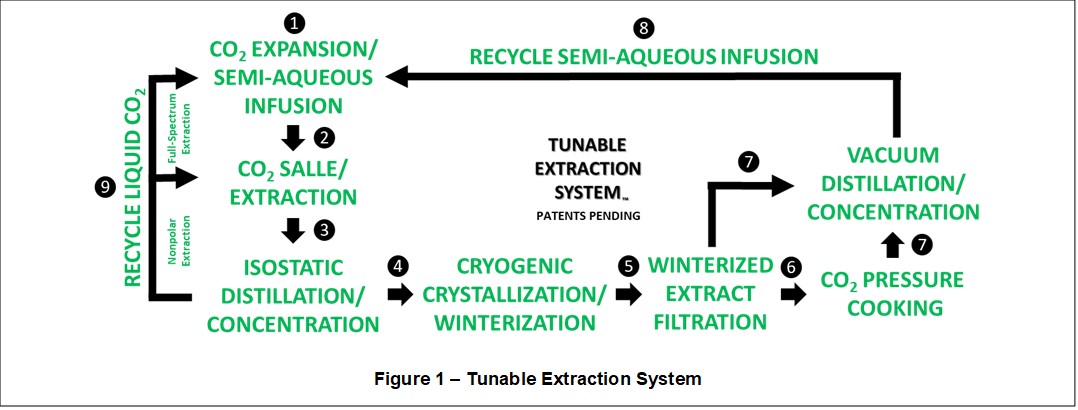
Unique and Essential Physicochemical Functions of the Infusion
The semi-aqueous infusion provides many essential and collaborative functions within the Tunable Extraction System, including:
1. Polar co-extractant and cellulosic membrane swelling agent ❷;
2. Autogenously cooled polar solvent trap for concentrating full spectrum extracts during liquid CO2 isostatic pressure distillation ❸;
3. Polar antifreeze agent and polarity shifting solvent during in-situ CO2 cryogenic crystallization and winterization ❹;
4. CO2-saturated polar liquid phase enabling winterized extract filtration ❺ to remove high molecular weight (HMW) nonpolar precipitates;
5. Reaction environment during CO2 pressure cooking ❻ to complete extract decarboxylation; and
6. A natural, safe, preservation solvent for full spectrum extract concentrates ❼.
Closed Loop and Selective Processing
Finally, the Tunable Extraction System is closed loop and provides selective processing. Semi-aqueous infusion ❽ and liquid CO2 ❾ are recovered and recycled in-situ during extract desolvation and concentration using vacuum distillation ❼ and isostatic pressure distillation ❸, respectively. Moreover, liquid CO2 ❾ may be used alone as a (polarity-directed) nonpolar extraction solvent ❷, by-passing the semi-aqueous infusion step ❶. This enables selective mixed-polarity and polarity-directed extractions. Following nonpolar liquid CO2 extraction ❷ and isostatic pressure distillation ❸, the Liquid CO2:Extract concentrate is infused prior to post-processing steps ❹ through ❼.
Polarity-Directed and Mixed-Polarity CO2 Extraction Solvent Systems
A Tunable Extraction System utilizes both polarity-directed and mixed-polarity CO2 extraction solvent systems, described as follows:
- 1. Polarity-directed extraction systems utilize nonpolar liquid phase CO2 (preferred) or a polar semi-aqueous solution (i.e., heated-pressurized water and additives). A semi-aqueous extraction process is followed by CO2 expansion/salting-out assisted liquid-liquid extraction (CO2 SALLE) to recover water extracted phytochemicals.
- 2. Mixed-polarity extraction systems provide a full spectrum (nonpolar, polar, and ionizing) solubility gradient (↕δ), utilizing liquid CO2 and semi-aqueous solutions containing water and one or more water-soluble or water-emulsifiable compounds, and used in a CO2 SALLE process. Mixed-polarity extraction processes can be performed within the CO2 solvent phase (dry extraction), the semi-aqueous solvent phase (wet extraction), or sequentially using both semi-aqueous and CO2 solvent phases (wet-dry extractions).
An important optimization in an extraction process is the swelling of cellulosic plant structures to improve solvent access and mass transfer. According to Zuorro (2020), plant celluloses are organized in microfibrils containing both crystalline and amorphous regions. Microfibrils are assembled into fibers of larger diameter that are cross-linked by hemicelluloses and embedded in a gel-like pectic matrix. Solvent molecules of small size, high polarity, and capable of participating in hydrogen bonding (as a donor, acceptor, or both) can penetrate the plant matrix and adsorb on hydroxyl groups. Following adsorption, some hydrogen bonds are broken, increasing the distance between the cellulose fibers, and causing the material to swell. In most cases, swelling is limited to the amorphous regions of cellulose, which are more reactive and accessible to solvent. Swelling solvents such as water (δSA=45.2 MPa1/2) and ethanol (δSA=21.3 MPa1/2) act as both hydrogen bond donors and acceptors.
As such, mixed-polarity solvent extraction systems containing nonpolar, polar, and ionizing (i.e., carbonic acid) compounds serve as swelling-extraction collaboration partners which provide more capable and efficient exhaustive extraction performance as compared to polarity-directed solvent extraction systems. Nonpolar, polar, and ionizing properties of each component in a Tunable Extraction System are individually expressed within the extraction solvent mixture. In this regard, relatively small amounts of polar (i.e., EtOH-H2O) and ionizing (i.e., H2CO3) co-extractants infused into liquid CO2 significantly improve the polar (δP) and hydrogen bonding (δH) solubility properties of the extraction solvent system, producing a solubility gradient (↕δ).
Liquid CO2 as a Carrier and Co-Extraction Solvent
As discussed in our blog article, Making the Case for Liquid CO2, liquid CO2 has consistently demonstrated superior exhaustive extraction performance as compared to supercritical CO2 in both simple and complex man-made and natural polymer extraction applications, and for a wide range of organic extractables. Polymeric swelling, membrane plasticization, solubility parameters, and CO2 expansion of liquids are important aspects in a liquid CO2 extraction process. Swollen and plasticized physical structures improve liquid CO2 (and co-solvent) penetration into pores. Moreover, CO2 expansion of co-extractants and extractable compounds enables improved mobility or mass transfer. Finally, liquid CO2 extraction process performance is dependent upon solvent-extract solubility parameter match-up, extractable molecular mass and physical state (solid vs. liquid), and co-solvency effects.
Ready for next steps? Try our CO2 Powered Consultation.









