Liquid CO2 is a Better Solvent
Higher Power, Capacity, and Speed
Laboratory testing and commercial equipment performance comparisons demonstrate that liquid CO2 is a better exhaustive extraction solvent than supercritical CO2. Liquid CO2 is superior in terms of higher solvent power, solvent capacity, and extraction speed, and particularly when coupled with extraction process intensification measures such as chemical modification, ultrasonics, and centrifugation.
CO2 Extraction Process Development (1985 to Present)
David Jackson, our company Founder and President, began his career in the CO2 development field in 1985 at Hughes Aircraft, Technology Support Division, evaluating solvent extraction capabilities and characteristics of so-called dense phase gases, which included primarily supercritical and liquid carbon dioxide (scCO2 and LCO2), but also incorporated limited investigations of supercritical and liquid nitrous oxide (scN2O and LN2O), supercritical argon (scAr), supercritical xenon (scXe), and several liquid Freon® refrigerants. The goal of the research and development program was to qualify dense phase CO2 (and other possible dense phase gas solvents) as replacements for ozone-depleting or toxic chlorofluorocarbon (CFC) solvents including Freon® 113, trichloroethylene (TCE), and perchloroethylene (PERC) used to prepare materials for use in Space environment.
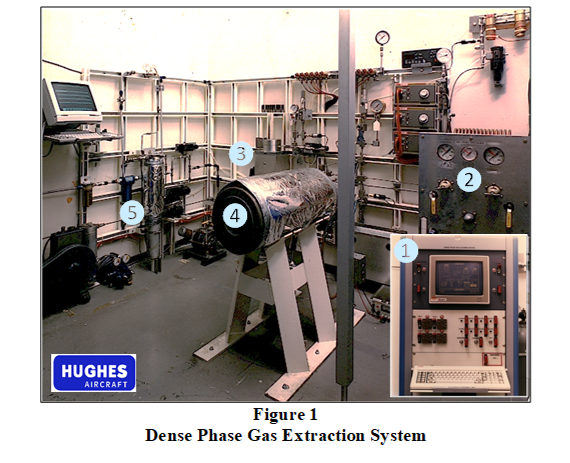
Extensive process development and performance testing was performed between 1985 and 1988 using a semi-automated dense phase gas extraction system shown in Figure 1, developed by Jackson and colleague, Mr. Orval Buck. The extraction system featured a PC-based monitoring and control system (❶), a dual-stage high pressure pneumatic pump system (❷), a temperature-regulated 1-liter extraction system (❸) rated for 5,000 psi, a temperature-regulated 5-liter extraction system (❹) rated for 30,000 psi, and a pressure- and temperature-regulated phase separation system (❺).
Supercritical CO2 extractions were performed at a (minimum) temperature of 305 K (32°C) and a pressure of 240 atm (3,500 psi). Liquid CO2 extractions were performed under supersaturation conditions at a (maximum) temperature of 298 K (25°C) and a pressure of 100 atm (1,500 psi) to mitigate observed lower dissolution performance under equilibrium liquid CO2 pressure-temperature conditions (i.e., 20-25°C and 800-900 psi), thought to be caused by the formation of stratified gas-liquid phases within pore cavities of test materials. Dense phase CO2 extraction processes using these standardized supercritical or supersaturated liquid conditions employed a 4-hour extraction period comprising four (4) to eight (8) fluidize-dwell-drain cycles (complete solvent exchanges), which was pre-determined to provide optimal extraction performance based on total mass loss (TML) and non-volatile residue (NVR) testing. In this regard, manufacturing materials and devices tested under this program were previously characterized using conventional extraction solvents and methods. For example, TML and NVR levels were quantified by Soxhlet (exhaustive) extraction using hexane, Freon® 113, TCE, or PERC, including polar organic solvent modifiers such as methanol and isopropanol.
Comprehensive compatibility testing was performed before and after extraction of polymeric and metallic materials and devices used in (or to produce) spacecraft, thermal vacuum test chambers, and military ground systems. Polymeric materials and devices included high voltage electrical cables, connector pin insulators, interfacial seals, and vibration dampeners. Metallic materials and devices included machined laser optical benches and porous sintered bronze bearings. In addition, manufacturing aids such as wood stick cotton tipped cleaning swabs, Texwipe® cleaning wipes, and various packaging and insulating films were processed and tested. Materials compatibility tests for polymeric devices comprised hardness, tensile strength, elongation, and compression. Moreover, processed substrates and surfaces were examined using various analytical chemical methods including FTIR, SEM/EDX, ASTM E595 TML and collectable volatile condensable matter (CVCM), and MIL-STD-1246 NVR.
As discussed, extraction processes comprised numerous pre-programmed fluidize-dwell-drain cycles over a 4-hour period, mimicking a Soxhlet extraction process. In addition, materials applications concerning surface preparation for bonding included the addition of small quantities of gas solvent modifiers including methanol, isopropanol, and hydrogen peroxide. Extractables included volatile and non-volatile, and non-ionic and ionic organic and inorganic compounds including oils, additives, fillers, colorants, plasticizers, and curing agents, which represented a broad range of Hansen Solubility Parameters (HSP). Extractables removed from test materials and devices were collected for analysis or disposal, and CO2 gas was exhausted to the atmosphere.
Among the several dense phase gases evaluated between 1985 and 1988, dense phase CO2 was qualified and selected as an approved solvent replacement. This was based on materials compatibility, environmental, health and safety, cost of operation, and extraction performance. In 1988, dense phase CO2 extraction was incorporated into a Hughes process specification for preparing materials for Space and vacuum environments. Early customers and applications (outside the Hughes organization) for the new dense phase CO2 extraction process included NASA Marshall Space Flight Center (MSFC) to extract organic residues from self-lubricating Armalon® (glass fiber reinforced PTFE) bearing cages used in liquid oxygen devices for the Space Shuttle program, and the U.S. Army to extract sintered bronze bearings for the M1 Abrams tank program.
Finally, several patents were issued from this foundational work and launched our 30-year CO2 commercialization effort beginning in the early 1990’s with the introduction of Centrifugal Liquid CO2™ extraction. Since that time, numerous commercial CO2 immersion and spray products have been developed with more than 40 patents issued worldwide.
Functional Polymer Extraction Performance
Notwithstanding liquid CO2 compatibility challenges (i.e., excessive swelling or depolymerization) for certain polymers, extraction tests performed under this dense phase CO2 process development program clearly demonstrated that liquid CO2 is a better extraction solvent than supercritical CO2 for most metallic and polymeric materials containing complex mixtures of solvent-extractable residues. Liquid CO2 consistently exhibited lower extract selectivity, higher solvent power, and higher solvent capacity, all of which decreased extraction time and resulted in a more complete extraction process.
Shown in Figure 2, various polymeric materials and devices were extracted to meet NASA E595 Total Mass Loss (TML) performance criteria. Extraction of polydimethylsiloxane vibration dampeners, wood-cotton swabs, and fluorosilicone connector insulators using supercritical CO2 and liquid CO2 under identical extraction periods showed that liquid CO2 could remove between 33% and 103% more extractable compounds.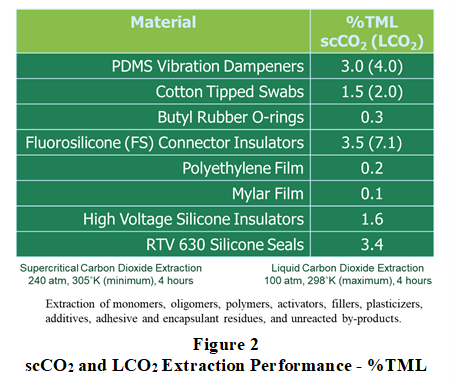
Moreover, shown in Figure 3, a study was performed comparing supercritical CO2 and liquid CO2 extraction of fluorosilicone insulators used in spacecraft multi-pin electrical cable harnesses. This polymer extraction study demonstrated that liquid CO2 could extract more residue, including ionic compounds, from the insulators. This was evidenced by a higher TML and a significant increase in column-to-column electrical resistance.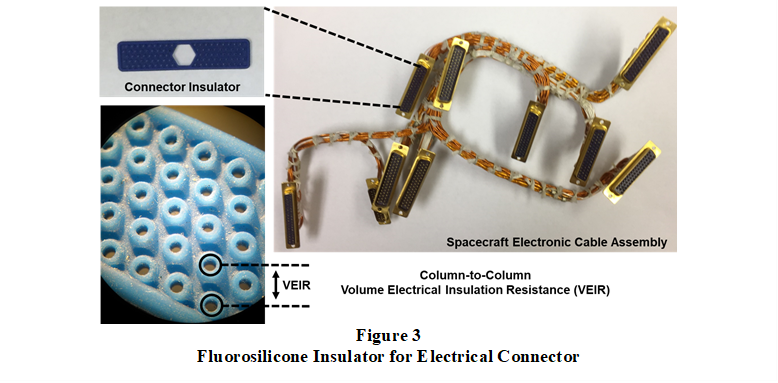
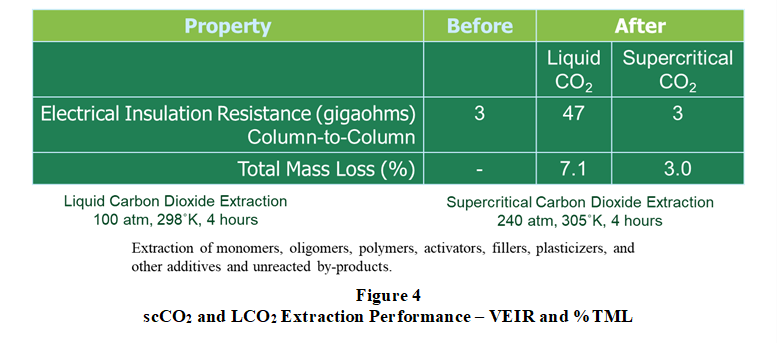
TML measurements based on ASTM E595, and volume electrical insulation resistance (VEIR) measurements based on ASTM D257 were performed on a batch of insulators (n>30) before and after standardized extraction procedures using liquid and supercritical CO2. Shown in Figure 4, liquid CO2 extraction produced connector pin insulators with an average volume electrical insulation resistance 16x greater than supercritical CO2. Moreover, insulators extracted with supercritical CO2 did not exhibit changes in electrical insulation resistance, indicating that no appreciable amount of ionic residue was extracted from the insulators.
Polymer swelling is a very important aspect of extraction performance. Swelling the polymer structure increases microscopic pore entrance diameters and cavity volumes which improves both solvent penetration and mass transfer. Liquid CO2 generally produces much higher swelling in polymers as compared to supercritical CO2. Observed differences in polymer swelling behavior and extraction performance between supercritical CO2 and liquid CO2 can be explained based on differences in Hansen Solubility Parameters (HSP). HSP for liquid and supercritical CO2 and two exemplary polymers are provided in Figure 5. Referring to Figure 5, liquid CO2 HSP are very similar to silicone and fluorocarbon polymers (as well as their internal extractable compounds). Due to like-seeks-like and like-dissolves-like cohesion energy principles, liquid CO2 significantly swells or depolymerizes polymers possessing similar
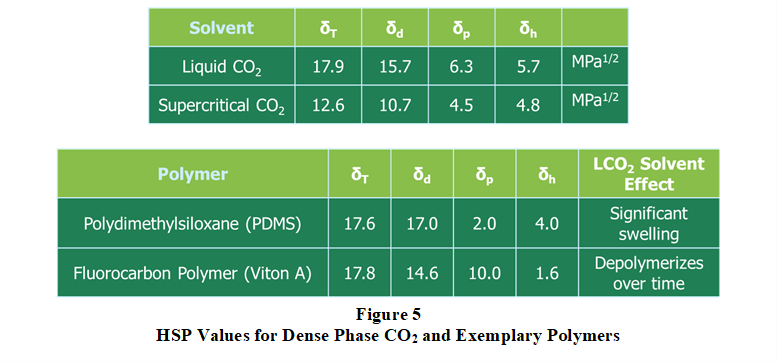
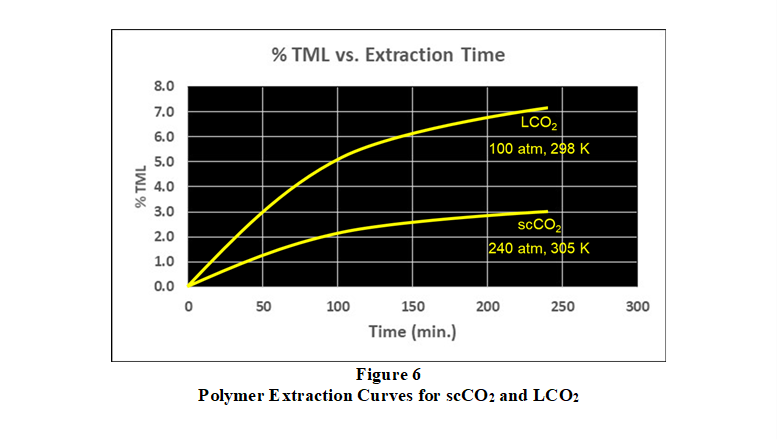
solubility parameters and results in a more complete extraction. In this regard, Figure 6 compares fluorosilicone polymer TML performance curves for supercritical CO2 and liquid CO2 extraction processes.
Liquid CO2 is a Better Extraction Solvent
Historical performance test comparisons demonstrate that liquid CO2 (and particularly supersaturated liquid CO2) is a better extraction solvent than supercritical CO2. Liquid CO2 is superior in terms of higher solvent power, solvent capacity, and extraction speed, particularly when coupled with extraction process intensification measures such as chemical modification, ultrasonics, and centrifugation. Moreover, liquid CO2 extractions are performed at lower temperatures and pressures, which saves energy and lowers capital equipment and operational costs. Figure 7 provides a comparison of key physical properties for supercritical CO2, liquid CO2, and ethanol (EtOH). In addition to HSP considerations for optimizing polymeric swelling, dissolution, and solubility phenomenon, the establishment and maintenance of density, viscosity, surface tension, and concentration gradients between the extraction fluid (solvent) and extract (solute) are key mass transfer performance objectives. In this regard and shown in Figure 7, liquid CO2 has a relatively high density (high fluid momentum transfer) with very low dynamic viscosity (low resistance to flow). 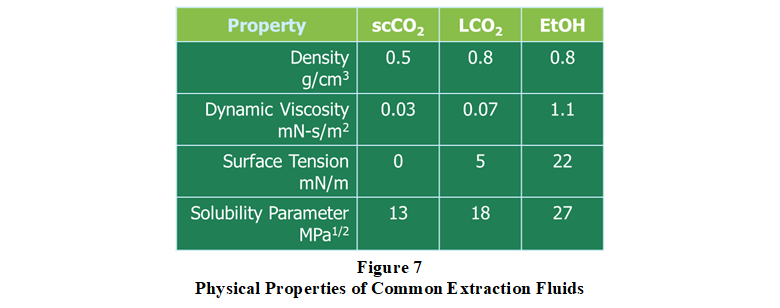 These factors coupled with a very low surface tension (high film forming and surface wetting ability) enhance the so-called Marangoni effect, a principal driver of solvent-solute interfacial mass transfer phenomenon. Given these considerations, besides solvent tunability for extract fractionation processes or extraction selectivity, there is no significant advantage gained using supercritical CO2. This experience and understanding were the principal rationale for the development and commercialization of Centrifugal Liquid CO2 in the early 1990’s.
These factors coupled with a very low surface tension (high film forming and surface wetting ability) enhance the so-called Marangoni effect, a principal driver of solvent-solute interfacial mass transfer phenomenon. Given these considerations, besides solvent tunability for extract fractionation processes or extraction selectivity, there is no significant advantage gained using supercritical CO2. This experience and understanding were the principal rationale for the development and commercialization of Centrifugal Liquid CO2 in the early 1990’s.
Centrifugal Liquid CO2 extraction technology was introduced in 1995 concurrent with successful technology demonstrations provided to the U.S. EPA and U.S. Air Force, as well as to California environmental agencies SCAQMD and California Regional Water Quality Control Board. These successful demonstrations generated significant interest from companies needing to replace soon-to-be-banned CFC cleaning solvents and processes. Figure 8 shows a Centrifugal Liquid CO2 extraction system installed at Gillette-PaperMate, Santa Monica, California (photographed in 2005). Installed and commissioned in 1998, this extraction system replaced an ultrasonic PERC solvent extraction system and process, providing clean processing, better machining oil extraction performance, higher processing capacity, and a lower cost of operation. The oil extraction rate was 2x faster and residual substrate oil levels (<2 micrograms/part) were as-good-as those achieved using the ultrasonic PERC solvent extraction process, and with a processing capacity of 250 lbs. of ball point tips (with swaged writing ball) every 20 minutes. Interestingly, prior to testing and qualifying the Centrifugal Liquid CO2 process beginning in 1997, Gillette-PaperMate investigated a supercritical CO2 extraction process with a third party. The supercritical CO2 process proved to be too slow and selective for certain components of the machining oil contaminating the miniature screw-machined ball point pen tips. 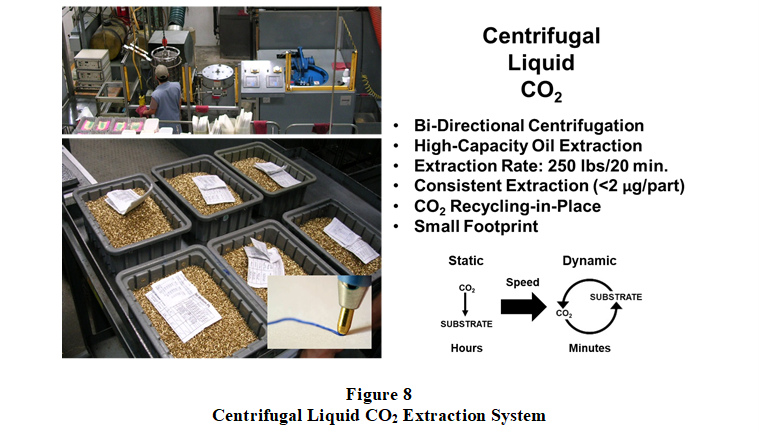 Moreover, part-to-part extraction quality was inconsistent for densely packed test batches, indicating both irregular solvent flow patterning and selective solubility. Centrifugal Liquid CO2 technology transformed dense phase CO2 extractions, providing cost- and performance-effective high-capacity extraction capability in a small footprint and reducing processing time from hours to minutes.
Moreover, part-to-part extraction quality was inconsistent for densely packed test batches, indicating both irregular solvent flow patterning and selective solubility. Centrifugal Liquid CO2 technology transformed dense phase CO2 extractions, providing cost- and performance-effective high-capacity extraction capability in a small footprint and reducing processing time from hours to minutes.
Past is Prologue
In 1985, the idea of using a dense phase gas as a cleaning agent to extract manufacturing materials and devices was novel and would launch 35 years of CO2 cleaning process innovation and commercialization. However, dense phase CO2 extractive cleaning processes developed in the 1980’s were ideated and modeled from prior art supercritical and liquid CO2 value extraction and fractionation processes. In this regard, a significant amount of prior art literature was studied to develop the initial knowledge base required to adapt this technology to materials preparation applications. For example, one of the first CO2 extraction processes studied employed liquid CO2 for treating, separating, and purifying an oil-bearing material and is described in a 1931 U.S. Patent No. 1,805,751, issued to E.B. Auerbach (Berlin, Germany), for a “Process for Treating, Separation, and Purifying Oils”. Many value extraction patents and papers like the Auerbach patent were studied and used to develop the original materials extraction and contaminant separation processes. Another excellent resource relied upon was a 1986 book entitled “Supercritical Fluid Extraction: Principles and Practice”, by McHugh and Krukonis.
Centrifugal Liquid CO2 extraction technology was introduced in the mid 1990’s. Early adopters included Allied Signal Aerospace, Therm-O-Disc Emerson Electric, Delphi Automotive, Pentel, Weldcraft, and Gillette-PaperMate, among others. These first cleaning installations involved relatively small and complex parts cleaning challenges such as oil and metal chip filled ball point pen tips with swaged writing balls, sandwiched and nested oily bimetal discs, and oily micro-ported fuel injector components. Compared to competing water-based or aqueous cleaning processes, Centrifugal Liquid CO2 proved superior in complex parts cleaning applications involving densely packed small parts possessing microscopic porosity, internal cavities, and sandwiched interfaces. However, the capital cost for large diameter high pressure CO2 immersion vessels needed for high capacity-large parts cleaning applications proved to be a competitive disadvantage. To address this constraint, we innovated CO2 Composite Spray™ cleaning technology, which bridged the parts cleaning capacity-size gap in precision cleaning applications. In this regard, we gave manufacturers the ability to transform large high volume and high cost-per-clean aqueous immersion cleaning operations into smaller, more selective, and more economical robotic CO2 spray cleaning operations for precision manufactured products such as hard disk drives, CMOS camera modules, electronic sensors, among many others.
Coming full circle, our latest CO2 innovations are focused exclusively on value extraction market and application opportunities like those that founded our journey in 1985. Over the years since our start, we have investigated several value extraction opportunities for Centrifugal Liquid CO2 technology. Investigations have included, for example, extracting dried and ground Korean grapefruit (for isolating Naringin), extracting residual soybean oil from pressed soybeans (for use in lubricants), and extracting oil from dried algae (for use as an energy source). None of these applications provided a compelling business proposition for our smaller capacity extraction systems, so we chose to stay focused on our primary mission in the precision cleaning market. Nevertheless, the value extraction market has evolved considerably over the past several years, for example with the recent enactment of favorable Cannabis and Hemp legislation and expanding consumer interest in natural products. Today, small to medium capacity extraction of high value natural products from plants presents an attractive economic model for our dense phase CO2 technology. The combination of the intrinsic commercial value of natural products and the increasing desire for natural, non-toxic, and non-contaminating processing technology to produce so-called “Clean Label” food and beverage additives provide a powerful value proposition for our dense phase CO2 technology. To address this emerging opportunity, we are developing and commercializing innovative small to high-capacity solutions for key value extraction markets and applications. We call our new CO2 program and technology platform Smart Extraction™.
Smart Extraction™ Technology
Smart Extraction™ technology is used in our SLO Cooker™ solid-liquid oil extraction system and FloraSPEC™ extraction process monitoring system and is a licensable CO2 intellectual property platform comprising interrelated proprietary and patents-pending natural product extraction, analytical chemical analysis, and emulsification processes. Our keystone extraction technology is a Tunable Extraction System™ driven by a dense phase CO2 Salting-out Assisted Liquid-Liquid Extraction (CO2 SALLE™) process. A Tunable Extraction System utilizes a semi-aqueous solution comprising water (a majority component) containing water-soluble or water-emulsifiable compounds and additives, for example an alcoholic beverage or any other application-customized semi-aqueous extraction formulation. The CO2 SALLE process is the engine that selectively drives the Tunable Extraction System. Very different from solvent blends or pressure- and temperature-tunable dense fluids such as supercritical CO2, a CO2 SALLE-driven Tunable Extraction System employs multiphasic, synergistic, and synchronistic surface tension gradients, density gradients, solubility parameter gradients, process intensification, and solvent phase shifting process for synthesizing polar and non-polar extracts from plants, foods, alcoholic beverages, subcritical water extractants, and environmental samples, as well as other liquids, solids, pastes, and slurries.
Select References
1. “Dense Phase Carbon Dioxide Cleaning Process: Liquid and Supercritical Carbon Dioxide as Cleaning Solvents”, D. Jackson, Hughes Aircraft Co., 10th Annual Aerospace Contamination Control Working Group Meeting, Draper Laboratories, Danvers, MA, May 1987
2. ASTM E595 – Total Mass Loss (%TML) and Collected Volatile Condensable Materials (CVCM) from Outgassing in a Vacuum Environment
3. ASTM D2240 - Standard Test Method for Rubber Property - Durometer Hardness
4. ASTM D412 - Tensile Strength Properties of Rubber and Elastomers
5. ASTM D575 - Standard Test Methods for Rubber Properties in Compression
6. U.S. Patent 5,013,366, “Cleaning Process using Phase Shifting of Dense Phase Gases”, D. Jackson and O. Buck
7. EPA-600/R-96-131, “Demonstration of a Liquid Carbon Dioxide for Cleaning Metal Parts”, November 1996
8. U.S. Patent 5,344,493, “Cleaning Process using Microwave Energy and Centrifugation in Combination with Dense Fluids”, D. Jackson
9. ASTM D257 - Standard Test Methods for DC Resistance or Conductance of Insulating Materials
10. Hansen Solubility Parameters, C. Hansen, 2nd Ed, CRC Press, 2007
11. “CO2 for Complex Cleaning”, D. Jackson, Process Cleaning Magazine, July/August 2009
Ready for next steps? Try our CO2 Powered Consultation.
<!-- Event snippet for Smart Extraction Blog Post - Liquid CO2 is a Better Solvent conversion page -->
<script>
gtag('event', 'conversion', {'send_to': 'AW-10785109592/7lZZCPaMwZEDENjs3pYo'});
</script>








