Solubility Gradients
Synergistic, Synchronistic, and Synthesizing
A plant is a complex system of physical structures, phytochemicals, and biochemical processes possessing a range of distinct solubility properties and behaviors. Physical structures and chemicals are logically organized and located within a plant and possess solubility parameter gradients, or more simply Solubility Gradients (↕δ). For example, plant structures possess solubility parameters ranging from hydrocarbon-like (Exterior Structures and Surfaces) to water-like (Interior Structures and Surfaces). More significantly, solubility gradients are present in synergistic, synchronistic, and synthesizing physicochemical processes such as photosynthesis, fermentation, nutrient transport, defense mechanisms, biochemistry, and biochemical processes.
Solubility Gradients are the inspiration for our Tunable Extraction System.
The Complex Plant System
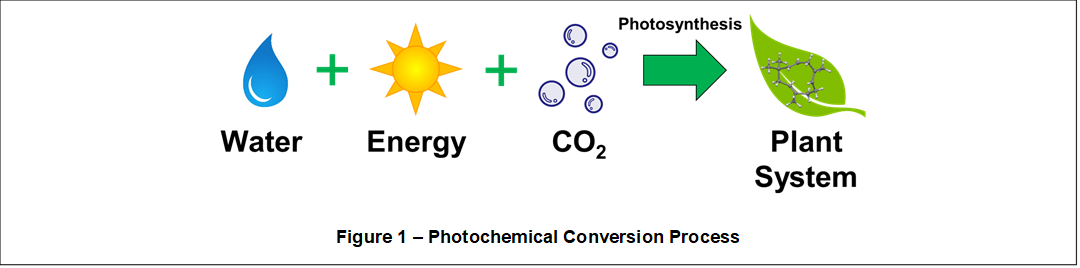
Plants are essential for any ecosystem. Deriving their energy from sunlight, they provide all the energy needed for the ecosystem to operate. Shown in Figure 1, a photochemical conversion process called photosynthesis, uses light energy from the sun in combination with atmospheric carbon dioxide (CO2) to form organic compounds. Daytime and nighttime respiration processes absorb and release oxygen (O2) and CO2. Plants require water (H2O) and organic chemical nutrients containing nitrogen (N), sulfur (S), and phosphorous (P). These fundamental chemicals and processes support numerous catalyzed metabolic operations to manufacture, integrate, and operate the highly organized plant system comprising interoperating biochemical production and processing factories within complex physical structures and organs.
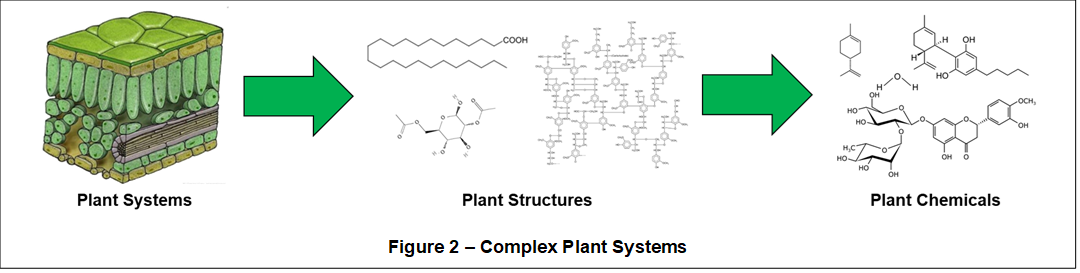
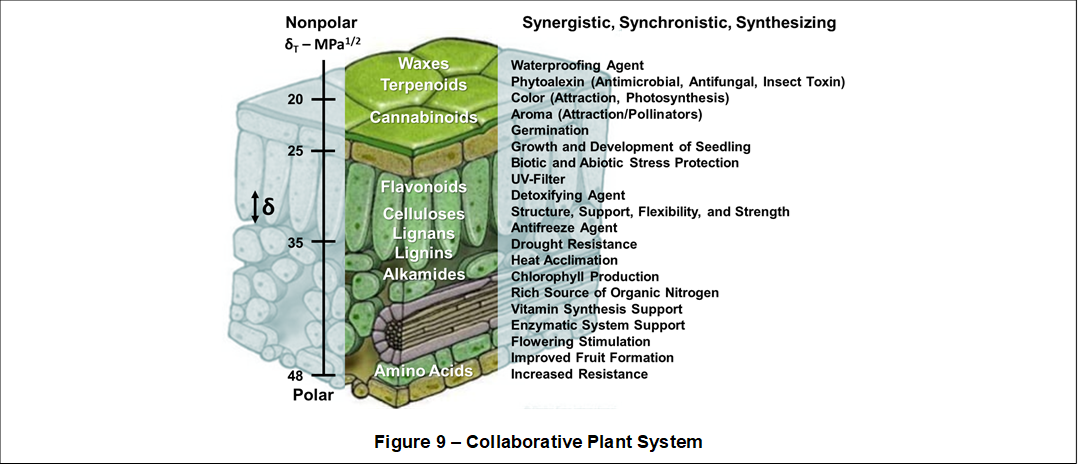
Shown in Figure 2, plant systems comprise various plant structures (i.e., cellulosic membranes and filaments) and a huge quantity of variously located (i.e., root, stem, leaf, flower) and distinct plant chemicals including, for example:
- - Waxes
- - Terpenoids
- - Cannabinoids
- - Flavonoids
- - Cellulose
- - Lignins
- - Alkamides
- - Amino Acids
As an example, Calvi et al. (2018) performed a multi-step exhaustive solvent extraction of Cannabis Sativa using several different solvents in series possessing a range of polarities.
It was determined in this study that Cannabis Sativa contains at least 554 identifiable compounds, among them 113 cannabinoids and 120 terpenoids.
Solubility Parameters
An effective method for simplifying our understanding of the vast and complex plant system, particularly with regards to developing an efficient and effective solvent extraction process, involves the use of the Solubility Parameter (δ). Solubility parameters for the various plant structures and chemicals are matched to solubility properties for various extraction solvents or solvent blends to formulate an optimal natural product extraction system. Systems possessing similar solubility parameters tend to integrate (i.e., dissolve, wet, swell) and systems with dissimilar solubility parameters tend to segregate (i.e., phase separate, repel).
Shown in Figure 3, solubility parameters provide a quantitative measurement of solubility behavior (dissolution, swelling, wetting, etc.) within plant structures, plant chemicals, and extraction solvent systems, in terms of dispersive energy (δD), polar energy (δP), hydrogen bonding energy (δH), and a very important subset called a specific association parameter (δSA). Calculated using the Hildebrand cohesive energy equation (δ=((ΔHv - RT)/V)1/2), solubility parameters are commonly quantified in the cohesive energy unit, MPa1/2, and range between 12 MPa1/2 and 48 MPa1/2 for natural product extraction systems.
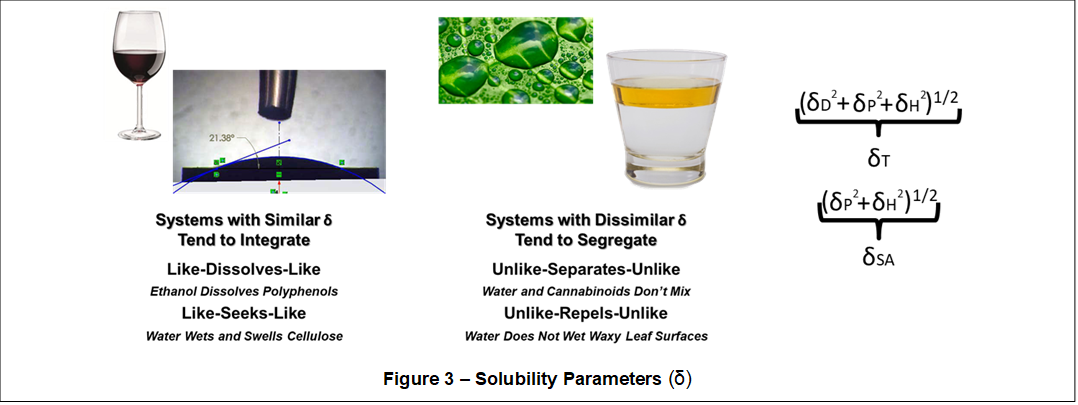
A total solubility parameter (δT) comprises the square root of the sum of squares of δD, δP, and δH. Given the self-similarity of dispersion (dipole-dipole) energies (δD) within a natural product extraction system, the specific association parameter (δSA) is an important differentiating measure of polarity and hydrogen bonding (hard-soft acid-base) characteristics and is defined as the square root of the sum of the squares of δP and δH.
Solubility Parameter Gradients
First noted by Khayet and Fernandez (2012), an interesting aspect of plant physical structures and surfaces is the presence of a Solubility Parameter Gradient, or more simply a Solubility Gradient (↕δ). Shown in Figure 4, external plant surfaces possess solubility parameters that are hydrocarbon-like (i.e., exterior cuticular waxes) and internal plant surfaces possess solubility parameters that are water-like (i.e., cellulosic cell walls).
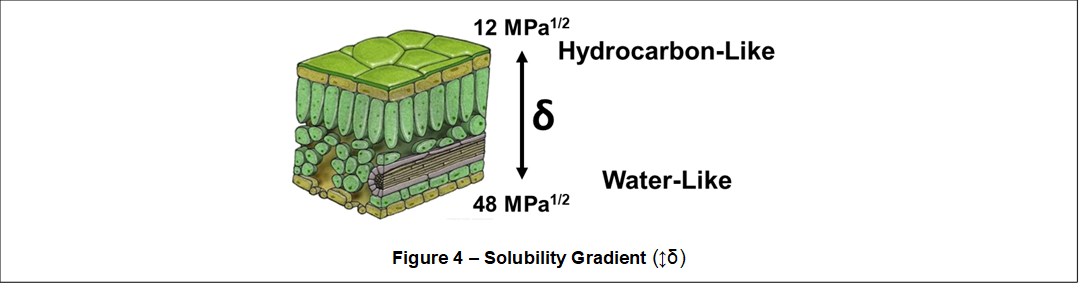
Investigating this aspect more deeply, it is discovered that Solubility Gradients also exist in the plant phytochemical systems and in chemical processes such as, for example, fermentation. Moreover, shown in Figure 5, there are numerous other physicochemical gradients such as molecular complexity, polar surface area, molecular weight, among
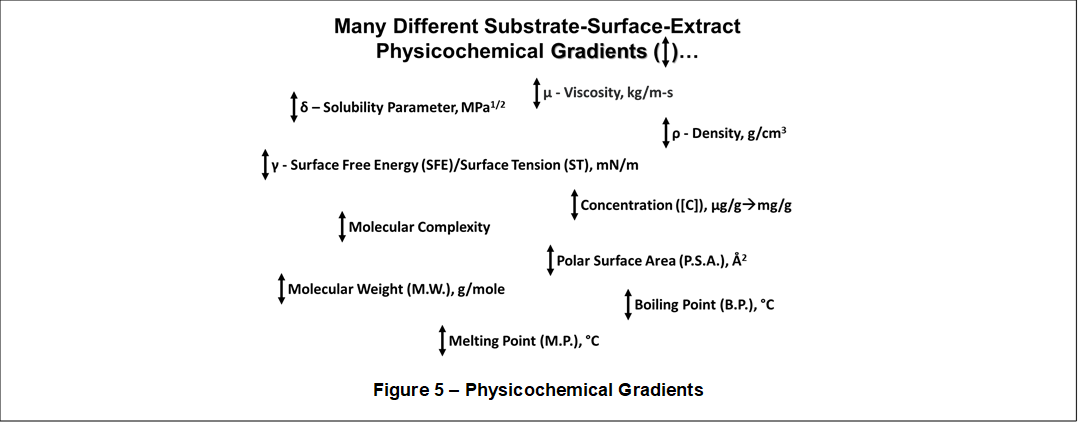
many others. Solubility parameters are related to all these physicochemical gradients.
This aspect is illustrated in Figure 6. A Solubility Gradient (↕δ) ranging between 12 MPa1/2 to 48 MPa1/2 contains numerous groups of similar plant chemistries including, for example, terpenoids, cannabinoids, and flavonoids. Also shown are physicochemical gradients for molecular complexity, polar surface area (P.S.A), and
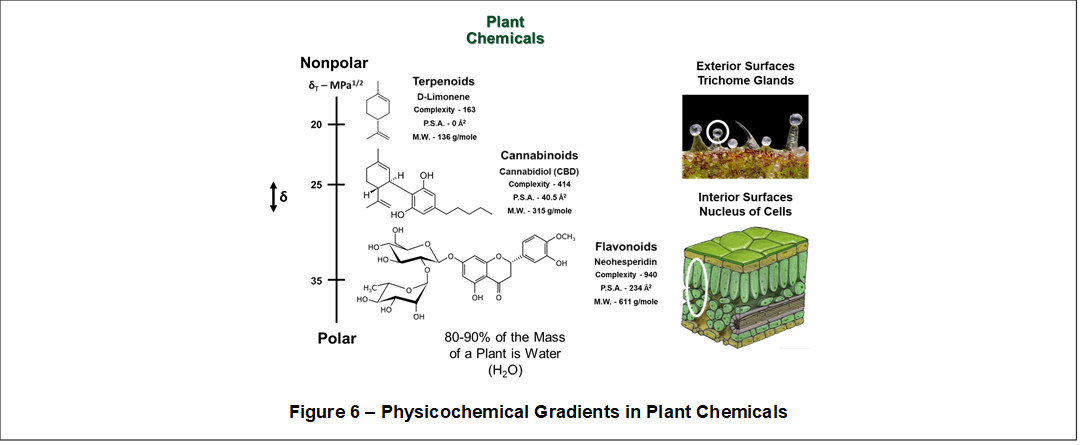
molecular weight.
Similar to plant chemicals and shown in Figure 7, a Solubility Gradient (↕δ) ranging between 12 MPa1/2 to 48 MPa1/2 contains numerous groups of similar plant structures including, for example, leaf surfaces and cell walls. Also shown in Figure 7 are physicochemical gradients for molecular complexity, polar surface area (P.S.A), and molecular weight.
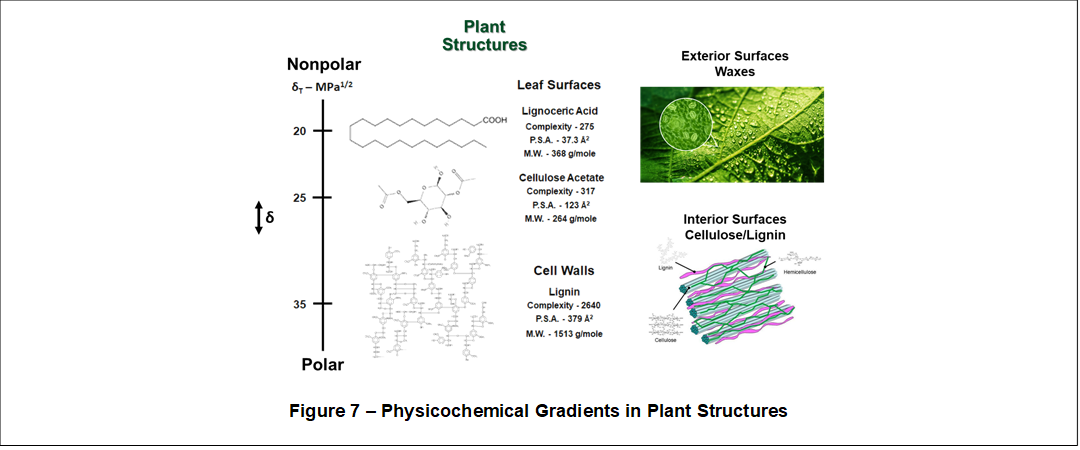
Moreover, shown in Figure 8, a Solubility Gradient (↕δ) ranging between 12 MPa1/2 to 48 MPa1/2 exists within a particular plant structure. Mota et al. (2017) showed that tree bark contains varying amounts and types of organic extracts based on the polarity of a solvent, using a so-called polarity-directed multi-step, multi-solvent extraction process.
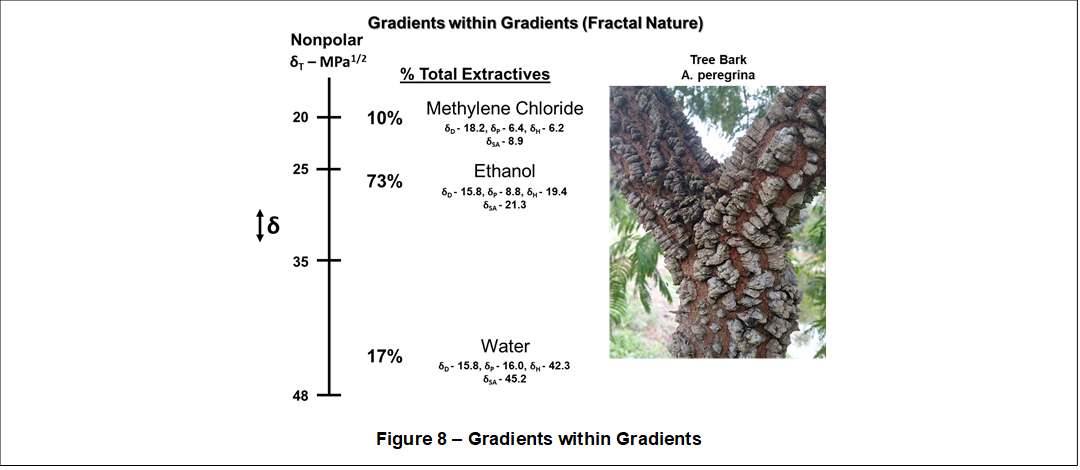
Synergistic, Synchronistic, and Synthesizing
Solubility Gradients are very useful physicochemical characteristics (biomarkers) that help simplify our understanding of complex plant systems. Solubility Gradients are manifested in many different plant structures and chemistries, working in synergy and synchronicity to synthesize other more complex plant structures and chemistries. Shown in Figure 9, a huge range of plant structures and chemistries collaborate to perform numerous critical plant functions.
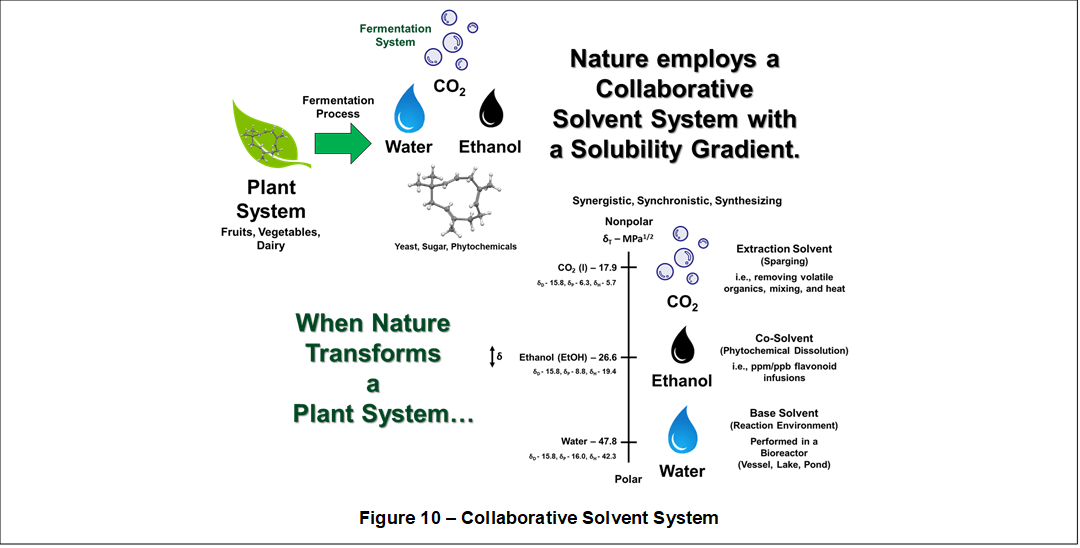
Finally, the apparent importance of a Solubility Gradient is also illustrated in plant biochemical processes. For example, when nature transforms a plant system in a fermentation process, a collaborative solvent system is produced which possesses a Solubility Gradient. Shown in Figure 10, water, ethanol, and carbon dioxide work collaboratively during the fermentation process to provide critical functions such as reaction environment, solvent modification, and removal of volatile by-products.
Tunable Extraction System™
As discussed above, there is a ubiquitous presence of Solubility Gradients in plant systems, structures, chemicals, and biochemical processes. The significance of this aspect is that there is no one perfect extraction solvent, regardless of its inherent chemical tunability (i.e., supercritical CO2, hydroethanolic solvent blends). As a result, numerous so-called polarity-directed solid-liquid extraction solvents and techniques are used today. With regards to exhaustive extraction, the academic literature is full of studies demonstrating that multi-step, multi-solvent extraction processes are required for efficiently extracting a true full spectrum of valuable natural products located in the various plant structures. However, conventional multi-step, multi-solvent processes waste time, space, energy, material, and labor inputs.
The Tunable Extraction System™ represents a new type of solid-liquid and liquid-liquid extraction approach inspired by Nature’s Solubility Gradient. Shown in Figure 11, the Tunable Extraction System combines a semi-aqueous solution comprising purified water and water-soluble or water-emulsifiable compounds, dense fluid (gas, liquid, or supercritical CO2), and a liquid or solid substance containing one or more extracts. The result is a highly tunable system providing adjustable chemical energy. Moreover, the Tunable Extraction System adapts to process intensification techniques such as heating, centrifugation, ultrasonics, and microwaves, which provide the necessary mechanical and thermal energy optimizations. As a result, the Tunable Extract System provides a fully adjustable Solubility Gradient ranging between 12 MPa1/2 and 48 MPa1/2.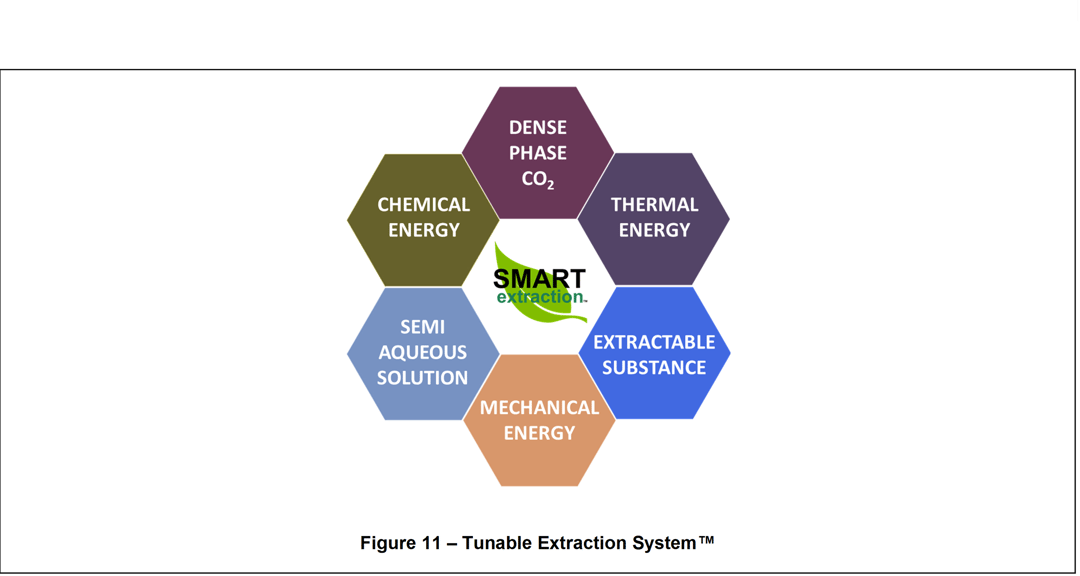
The Tunable Solvent Extraction System employs synergistic and synchronistic surface tension gradients, density gradients, solubility parameter gradients, process intensification, and dense phase CO2-driven solvent phase shifting for synthesizing non-polar, semi-polar, and polar extracts from plants, foods, beverages, soils, minerals, animal tissues, industrial wastewaters, and environmental samples.
Ready for next steps? Try our CO2 Powered Consultation.









